Calcite
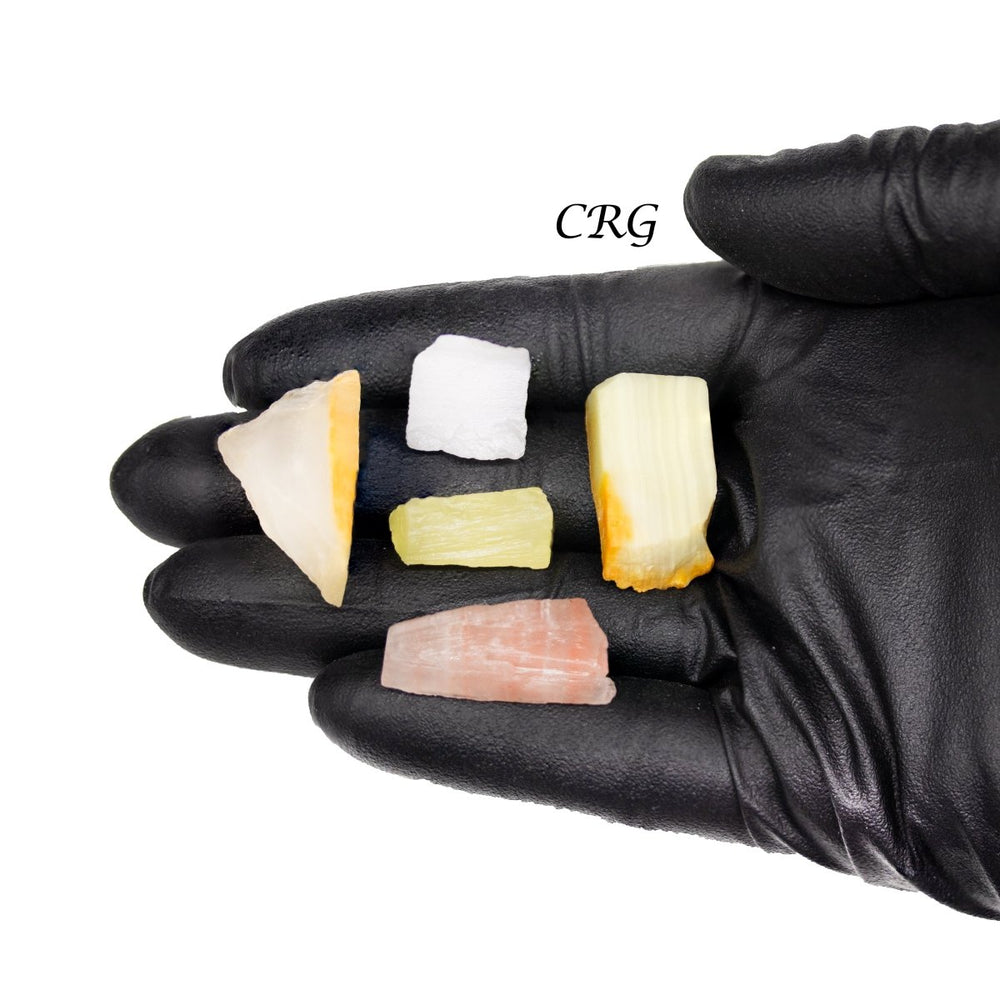
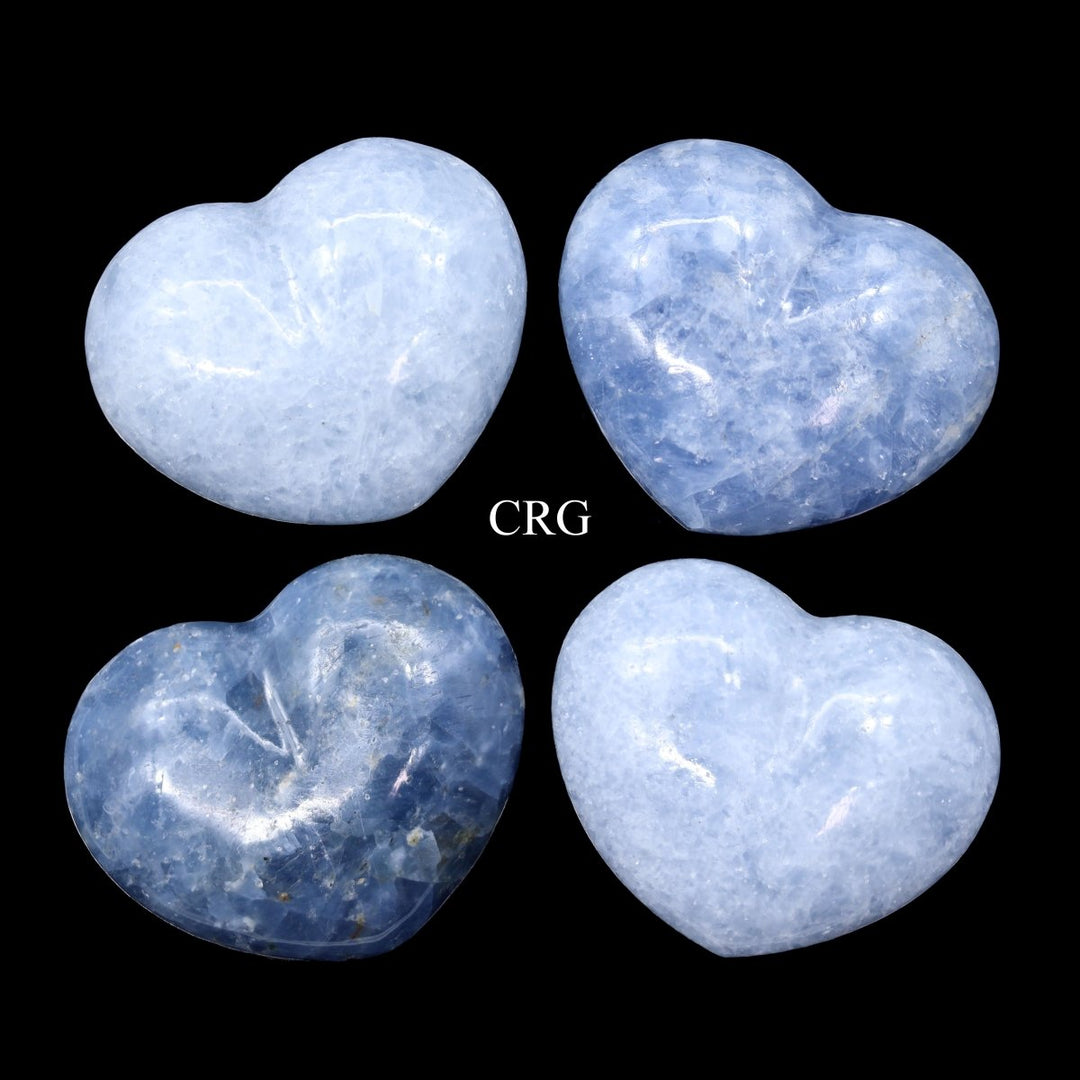
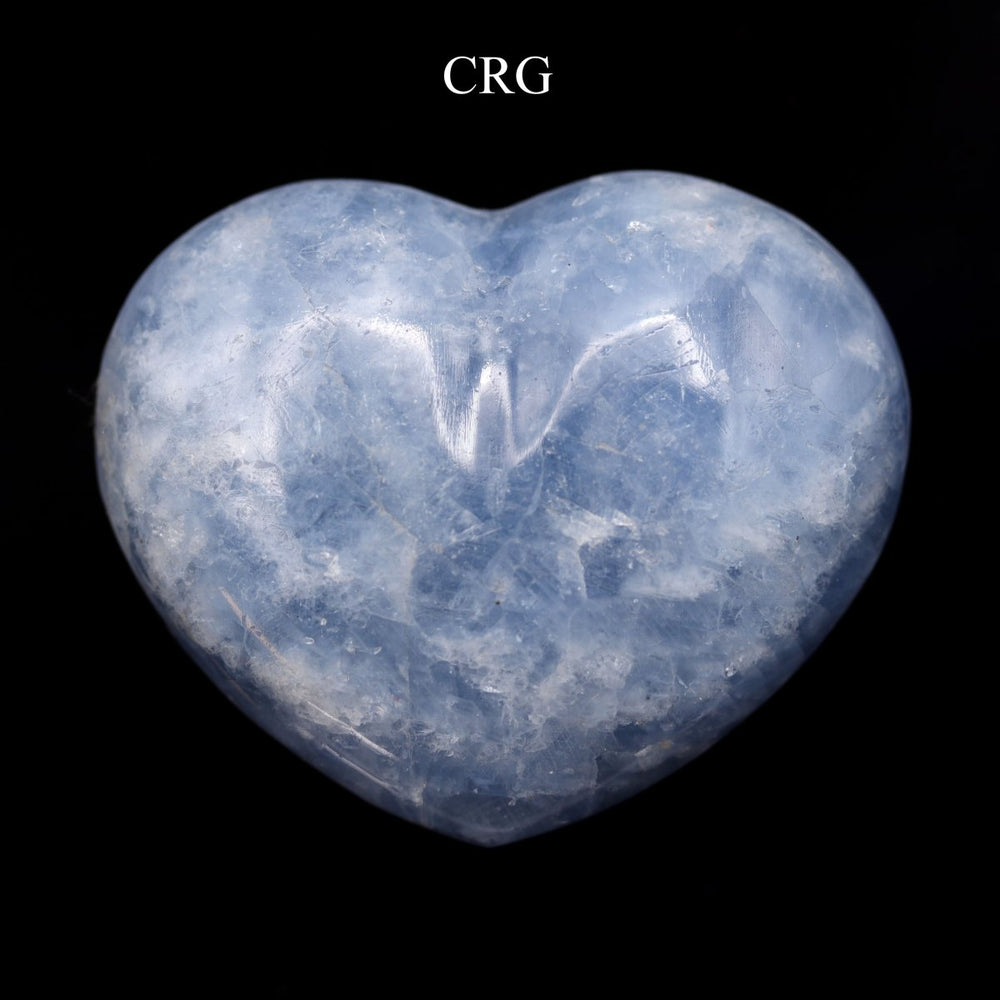
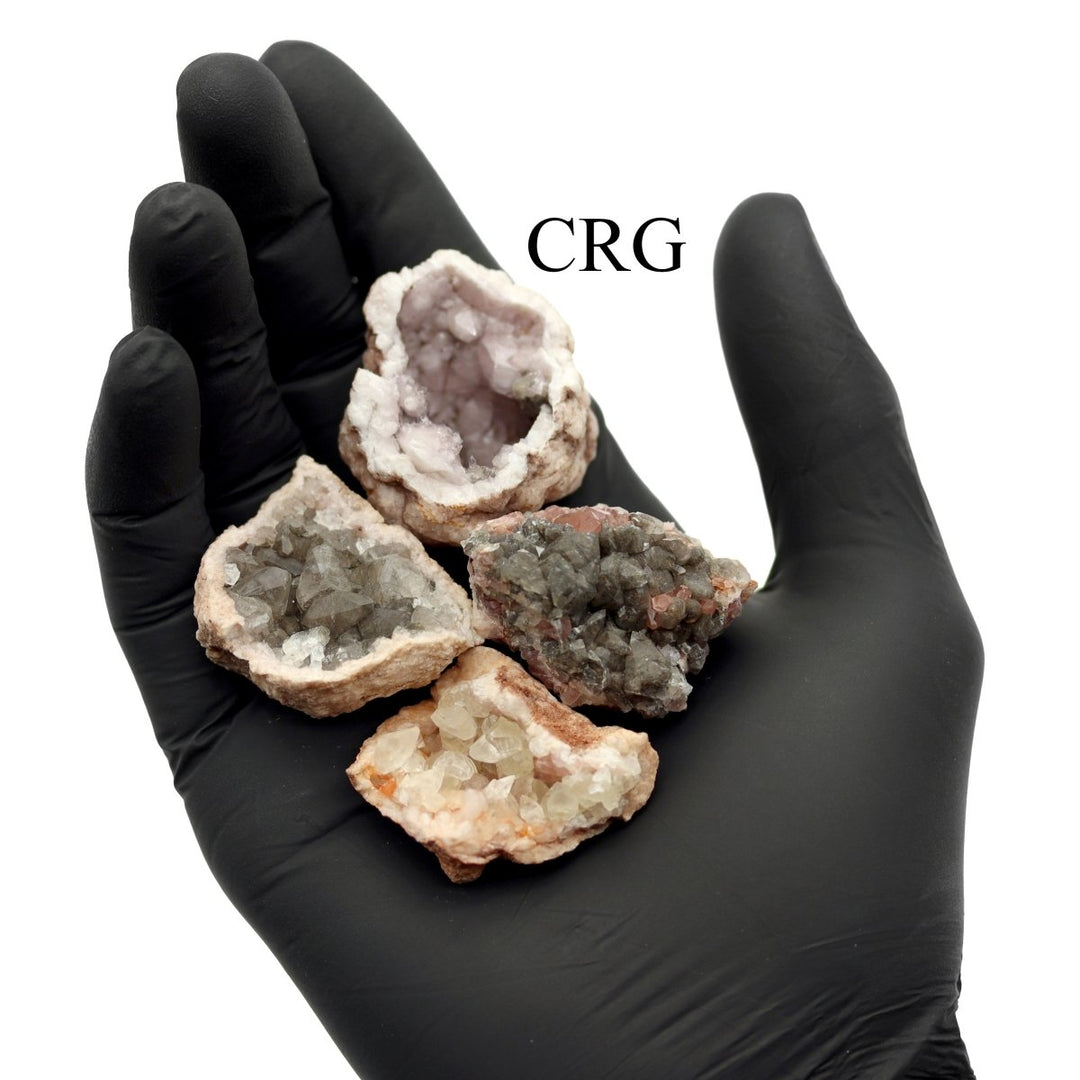
Calcite is primarily calcium carbonate (CaCO₃) and is the primary component of sedimentary limestone and metamorphic marble. Calcite forms in a wide variety of geological settings. It can precipitate directly from calcium-bearing solutions in marine and freshwater environments, form from biological processes in shallow seas (creating limestone from the shells of marine organisms), and appear in the veins and cavities of igneous rocks due to hydrothermal processes. One of the most distinctive features of calcite is its birefringence, or double refraction. You will see it doubled when looking through clear calcite at text or an object. It also has a Mohs hardness of 3, which makes it relatively soft and easy to scratch. Calcite has a vitreous to pearly luster and can exhibit fluorescence under UV light. Calcite is known for its incredible variety of shapes and colors, including clear, white, gray, red, green, blue, yellow, brown, orange, pink, and black. Impurities in the mineral's crystal structure cause these colors, and the mineral can also be transparent or translucent. Beyond being a major component of limestone and marble used extensively in construction and sculpture, calcite is also crucial in cement manufacture and as a balance in soil for agriculture due to its ability to neutralize acids. Its optical properties make it useful in various scientific instruments, and it is also popular among collectors and for decorative items. While calcite is found all over the world, notable deposits can be found in Mexico, Iceland, the United States, and the United Kingdom. Iceland spar, a clear and particularly perfect variety of calcite known for its optical properties, was historically significant in scientific research.